(Somatropin (INN) recombinant DNA origin, Escherichia coli.)
Consumer Medicine Information
What is in this leaflet?
Please read this leaflet carefully before you use NutropinAq.
This leaflet answers some common questions about NutropinAq. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using NutropinAq against the benefits he/she expects it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What is NutropinAq?
The active substance of NutropinAq is somatropin. Somatropin is human growth hormone, which is made by genetic engineering using the bacterial micro-organism Escherichia coli. The structure of somatropin is identical to human growth hormone of pituitary origin.
Somatropin has effects that are equivalent to human growth hormone of pituitary origin. Growth hormone exerts significant effects directly on the production of other hormones, e.g. IGF-1, and on metabolic actions. The anabolic and growth-promoting effects of somatropin are in some part indirect effects mediated by IGF-1.
What NutropinAq is used for?
NutropinAq is used for:
- Long-term treatment of children with growth failure due to inadequate endogenous growth hormone secretion.
- Long-term treatment of growth failure associated with Turner syndrome.
- Treatment of prepubertal children with growth failure associated with chronic renal insufficiency up to the time of renal transplantation.
- Replacement of growth hormone in adults with growth hormone deficiency originating in either childhood or adulthood.
Ask your doctor if you have any questions why NutropinAq has been prescribed for you. Your doctor may have prescribed it for another reason.
Before you use NutropinAq
Do not use NutropinAq:
- in case of hypersensitivity to somatropin or any of the other ingredients
- for growth promotion if growing is already finished
- if an active tumour (cancer) is present. Tell your doctor if you have or have had an active tumour. Tumours must be inactive and you must have finished your anti-tumour treatment before you start your treatment with NutropinAq.
- during acute critical illness due to complications following open-heart or abdominal surgery, multiple accidental trauma or in case of acute respiratory failure.
Treatment with NutropinAq should be discontinued if evidence of tumour growth develops.
Treatment with NutropinAq should be initiated and monitored by adequately experienced physicians.
Before you start to use it
Tell your doctor if:
- you have diabetes
- you have been diagnosed with Prader-Willi Syndrome
- you have long term kidney disease
- you have diabetes and worsening or severe eye disease
- you have problems with your thyroid
- you have any other hormone problems
- you are on a controlled sodium diet. NutropinAq contains 8.2mg of sodium per cartridge.
If you have had a tumour (cancer) in the past, especially a tumour affecting the brain, your doctor should pay special attention and examine you regularly for a possible return of the tumour. A small number of growth hormone deficient patients treated with growth hormone have had leukaemia (blood cancer). However, no cause and effect relationship with growth hormone treatment has been proven.
There may be an increased risk of developing an inflammation of the pancreas (pancreatitis) in children compared to adults treated with growth hormone. In case of severe and persistent abdominal pain, consult your doctor.
While you are using NutropinAq
Please ask the doctor for advice if you develop a limp or if you experience hip or knee pain.
If you have diabetes mellitus, please consult the doctor regularly during treatment with NutropinAq. Your insulin dose may require adjustment after treatment with NutropinAq is started.
Tell the doctor if you experience any visual changes, headache, nausea and/or vomiting, especially within the first eight weeks after starting treatment with NutropinAq.
Scoliosis (abnormal curve of the spine) may progress in any child during rapid growth. Your doctor should monitor you for any signs of scoliosis during treatment.
If you undergo a kidney transplant, NutropinAq treatment should be stopped.
If you are taking replacement therapy with glucocorticoids (steroid hormone medicines used to treat allergies, asthma, autoimmune diseases, or your body’s response to infection (sepsis)) you should consult your doctor regularly as you may need adjustments of your glucocorticoid dose.
Your doctor may take blood tests during treatment to check your blood count.
Pregnancy:
Treatment with NutropinAq should be discontinued if pregnancy occurs. Please ask your doctor or pharmacist for advice before taking any medicine.
Breast-feeding:
It is not known whether NutropinAq is excreted in human milk. However, absorption of intact protein from the gastrointestinal tract of the infant is unlikely. Please ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines:
No studies on the effects of NutropinAq on the ability to drive and use machines have been performed.
NutropinAq has no known effect on the ability to drive or to use machines.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription at your pharmacy, supermarket or health food shop.
NutropinAq and some medicines may interfere with the actions of each other, as listed below:
- Treatment at the same time with glucocorticoids may reduce the growth-promoting effect of NutropinAq
- NutropinAq may reduce insulin sensitivity, therefore patients with diabetes mellitus may require adjustment of their anti-diabetic therapy
- During administration of NutropinAq at the same time as corticosteroids, sex steroids, anticonvulsants or cyclosporin, the effect of treatment with these medicines may be affected. Please ask the doctor for advice
- In women taking oral estrogen replacement (hormone replacement therapy (HRT)) at the same time, the doctor may need to increase the dose of NutropinAq. Also, if a woman on NutropinAq stops oral estrogen therapy, the dose may need to be reduced. Ask your doctor for advice.
- Previously undiagnosed adrenal insufficiency may become apparent and require steroid treatment. Patients already treated for adrenal insufficiency may require adjustment of dose during NutropinAq treatment.
Your doctor or pharmacist has more information on medicines to be careful with or avoid while using this medicine.
How to use NutropinAq
How much and how often is it given?
The doctor will advise you about the individualised dose of NutropinAq. Please do not change the dosage without consulting the doctor or pharmacist. In general the dosage will be calculated according to the following rules:
Growth failure in children due to inadequate growth hormone secretion:
0.025 – 0.035 mg/kg bodyweight given as a daily subcutaneous injection.
Growth failure associated with Turner syndrome:
Up to 0.05 mg/kg bodyweight given as a daily subcutaneous injection.
Growth failure associated with chronic renal insufficiency:
Up to 0.05 mg/kg bodyweight given as a daily subcutaneous injection.
Treatment with NutropinAq may be continued up to the time of renal transplantation.
Growth hormone deficiency in adults:
Low initial doses of 0.15 – 0.3 mg given as a daily subcutaneous injection. The dose may be increased stepwise by the doctor according to the patient’s individual requirements. The final dose seldom exceeds 1.0 mg/day. In general, the lowest efficacious dose should be received. For older or overweight patients lower doses may be necessary.
NutropinAq is designed for use only with the NutropinAq Pen. The product is for use in one individual only.
Please administer the prescribed dose of NutropinAq solution for injection subcutaneously each day and change the site of injection each time. At the start of treatment it is recommended that a doctor or a nurse give the injection. After training the injection can be given by the patient or his/her carer. The medicine is supplied in a cartridge as a sterile solution with preservative for multiple use. For each single injection please use a new, sterile injection needle. Do not use the solution unless it is clear and not cloudy. Please see also the instructions for use.
Treatment with NutropinAq is a long-term therapy, for further information please ask the doctor.
If you forget to use it
After missing an individual dose a double dose should not be injected. The prescribed dosage regimen should be continued.
If you use more than you should (overdose)
If more NutropinAq than recommended was injected, please consult the doctor.
Acute overdose could lead initially to a glucose decrease (hypoglycaemia) and subsequently to a glucose increase (hyperglycaemia). Long-term overdose may result in an enhanced growth of ears, nose, lips, tongue and cheekbone (gigantism and/or acromegaly). These signs are consistent with the known effects of excess in human growth hormone.
Effects when treatment is stopped:
A disruption or early ending of the treatment with NutropinAq may impair the success of the growth hormone therapy. Please ask the doctor for advice before stopping the treatment.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using NutropinAq.
Like all medicines, NutropinAq can have side effects. Generally these are mild but you may need medical attention if you get some of the side effects.
Very common side effects (affecting more than 1 patient in 10):
Accumulation of fluids in the body (oedema) with swelling of the hands and feet (peripheral oedema), muscle pain (myalgia) and pain in one or more joints (arthralgia) in adults.
Common side effects (affecting between 1 and 10 patients in 100):
- Feeling of weakness (asthenia)
- Injection site reactions
- Headache
- Underactivity of the thyroid gland (hypothyroidism)
- Increased muscle tone (hypertonia)
- Impaired glucose tolerance
- Development of antibodies to the protein somatropin
- Pain in one or more joints (arthralgia), muscle pain (myalgia), accumulation of fluids in the body (oedema) and swelling of the hands and feet (peripheral oedema) in children.
Other common side effects which are specific to the disease which NutropinAq is being used to treat are listed below:
Children with growth failure due to growth hormone deficiency:
- Tumours, including malignant tumours, have occurred, particularly in patients previously diagnosed with cancer.
Children with growth failure associated with Turner Syndrome:
Common:
- abnormally heavy bleeding at menstruation (menorrhagia)
Children with growth failure due to chronic renal insufficiency:
Common:
- Renal failure (kidney dysfunction)
- peritonitis (inflammation of the lining of the abdomen)
- bone necrosis
- increase in creatinine blood levels.
Children with chronic renal insufficiency were more likely to develop increased pressure in the brain (intracranial hypertension).
Adults with growth hormone deficiency:
Very common
- abnormal sensations of tingling, pricking or numbness (paraesthesia).
Common:
- Increase in blood glucose (hyperglycaemia)
- increase in blood lipids (hyperlipidaemia)
- sleeplessness (insomnia)
- joint disorders, arthrosis (degenerative joint disease)
- muscle weakness
- back pain
- breast pain, breast enlargement (gynaecomastia).
Other side effects not listed above may also occur in some people.
If any side effects are noticed which are not mentioned in the leaflet, please inform the doctor or pharmacist.
If any side effect becomes troublesome or causes concern, tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital. You may need medical attention.
How to store NutropinAq
Storage
Keep out of the reach and sight of children.
Store at 2°C-8°C. Do not freeze.
Keep the container in the outer carton. Protect from light.
After first use, the cartridge may be stored for up to 28 days at 2°C-8°C. Do not remove the cartridge that is being used from the NutropinAq Pen between injections.
Do not use after the expiry date stated on the label of the cartridge and the carton.
Do not use NutropinAq if you notice that the solution is cloudy.
Disposal
If your doctor tells you to stop treatment or the medicine has passed its expiry date, ask your pharmacist what to do with any medicine that is left over.
Product Description
What it looks like
NutropinAq 10 mg (5mg/mL) solution for injection in a cartridge.
NutropinAq is a solution for subcutaneous use. The clear, colourless, sterile solution for multidose use is contained in a cartridge of glass, closed with a rubber stopper and a rubber seal.
NutropinAq is available in pack sizes of 1 and 3 cartridges.
Ingredients
Each cartridge of NutropinAq contains 2 mL of solution with a total of 10 milligrams corresponding to 30 International Units (IU) of the active substance somatropin.
The other ingredients are sodium chloride, phenol, polysorbate 20, sodium citrate, citric acid anhydrous and water for injections
Further Information
If you have any further questions on your NutropinAq treatment, or are unsure of the information, please see your doctor, who will be able to assist you.
Sponsor
Ipsen Pty Ltd
Level 5
627 Chapel Street
South Yarra Victoria 3141
Australian Registration Number (AUST R):
116678 (NutropinAq 5mg/mL)
134206 (NutropinAq Pen)
Date of preparation of this leaflet.
January 2023
NutropinAq Pen
Instructions for use with NutropinAq
DO NOT INJECT THE MEDICINE UNTIL YOUR DOCTOR OR NURSE HAS THOROUGHLY TRAINED YOU IN THE PROPER TECHNIQUES.
Caution:
Before using your NutropinAq Pen, please read the following instructions carefully. We also suggest you consult your doctor or nurse for a demonstration.
The NutropinAq Pen is designed for use only with cartridges of NutropinAq (for subcutaneous use only). Only use the pen needles recommended by your doctor or nurse.
The dosage scale located beside the window of the cartridge holder should not be used as a dose measurement. It should only be used to estimate the dosage remaining in the cartridge. Always refer to the LCD (Liquid Crystal Display), not audible clicks, for setting an injection of NutropinAq. The clicks are only audible confirmation that the black dose knob has been moved.
Always store the pen and cartridges in a clean, safe place in the refrigerator at a temperature between 2-8°C and out of children’s reach.
Protect from intense light. Use a cooler to store your NutropinAq Pen when travelling. The NutropinAq is designed to withstand a nominal (one hour maximum) period of time outside of the refrigerator on a daily basis. Avoid areas of extreme temperature. Check the expiry date of the cartridge before use.
To guard against the spread of infection, follow these safety measures:
- Wash your hands thoroughly with soap and water before using your pen.
- Clean the cartridge rubber seal with an alcohol swab or cotton ball saturated with alcohol.
- Avoid touching the cartridge rubber seal at all times.
- If you accidentally touch the cartridge rubber seal, clean it with an alcohol swab.
- The product is for use in one individual only.
- Use needles only once.
NutropinAq Pen Components:

Shown above are the items necessary for giving an injection. Gather all the pen components prior to use.
Your NutropinAq will be supplied separately.
Part I: Preparing and Injecting
Follow the instructions in this section if you are using the pen for the first time or are replacing an empty cartridge.
Inspect all new cartridges prior to use. Occasionally, after refrigeration, you may notice that small colourless particles are present in the NutropinAq solution. This is not unusual for solutions containing proteins like NutropinAq and does not affect the strength of the product. Allow the cartridge to come to room temperature and gently swirl. Do not shake. If the solution is cloudy or hazy or contains any solid matter, the cartridge should not be used. Return the cartridge to your pharmacist or prescribing doctor.
- Remove the green pen cap and unscrew the cartridge holder from the pen. If necessary, remove the empty cartridge and discard it properly.
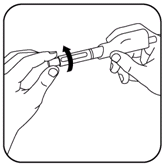
- Press the white reset button.
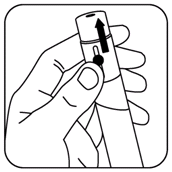
- Turn the black dose knob counter-clockwise back to its starting position until it no longer turns. (See illustration).
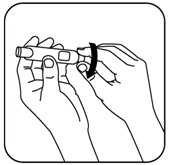
Then turn the dose knob clockwise until the first click position is reached (approximately 1/4 turn). This ensures that the plunger push rod is reset to the starting position. If this is not done when the dosage knob is first pushed in, NutropinAq will be wasted or the cartridge may crack.
- Insert cartridge into the cartridge holder, then screw the cartridge holder back onto the pen. (Be careful not to touch the rubber seal.)
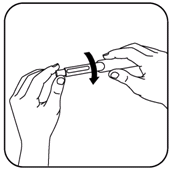
- Remove the paper seal from a new needle assembly and screw it onto the cartridge holder.
- Carefully remove both protective caps from the needle by pulling gently. Do not throw the larger cap away as it will be used later for proper needle removal and disposal.
- Holding the pen with the needle pointing upward, gently tap the cartridge holder to move any air bubbles to the top. While still holding the pen in the upright position, push in the black dose knob until it clicks into position. You should see a drop of solution appear.
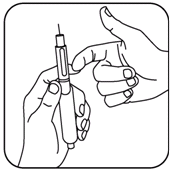
Be patient. If medicine doesn’t appear within a few seconds, you may need to push the reset button again.
- If no drop of medicine appears, push the white reset button again. Now turn the black dose knob clockwise (see illustration) by one click (0.1 mg). If you accidentally turn it too far, go back one click (0.1 mg).
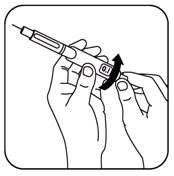
- While still holding the pen in the upright position, push in the black dose knob again and watch the needle tip for a drop of medicine to appear. Repeat steps 8 and 9 until it appears.
- Press the white reset button. (The digital dose display will blink for 5 seconds.)
- Set the required dose by turning the black dose knob.
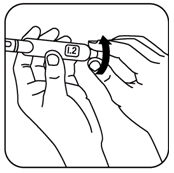
If you cannot dial the full dose, either start a new cartridge (as described in Part I), or inject the partial dose. Then, start a new cartridge (as described in Part I) to administer the remaining portion of your medication. Your doctor or nurse will advise you on the procedure for administering the last dose in the cartridge.
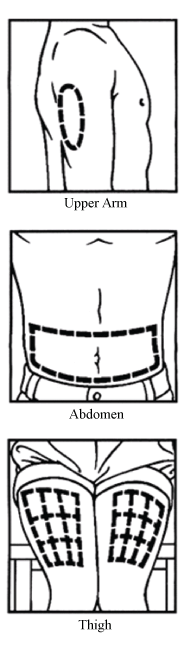
Prepare the injection site by wiping with an alcohol swab. Injection sites include the upper arms, abdomen, and upper thighs. Change the injection sites to avoid discomfort. Even if you develop a preference for one site, you still should rotate the injection site.
- If you are using the passive shield (or no shield) proceed to step 13.
If you are using the active shield, slide the shield onto the pen and push the 2 black lock knobs on the needle shield toward the tip.
- Set the tip of the pen on the prepared injection site and press the needle into the skin by pushing the pen downward until the shield is totally depressed. Your doctor or nurse will show you how to do this. Now you are ready to administer the dose. Press down on the black dose knob. Wait 5 seconds after the button is pushed, then withdraw the pen from the skin. A drop of blood may appear. Put a plaster on the injection site if you wish.
- Pull the needle shield off the pen (if you have used one) and place the larger needle cap on a flat surface. Slide the needle in to pick it up and push the cap completely down over the needle. Twist off the needle and discard it properly. Your doctor or nurse will tell you how to dispose of the items you have used for the injection. Always store your disposal container out of the reach of children.
- Attach the pen cap and return it to its case with the black dose knob pressed in. You should always store the pen in a refrigerator. Do not remove cartridge between injections.
DO NOT FREEZE.
For subsequent injections with the NutropinAq Pen, attach a new needle, push the white reset button and dial your dose.
Part II: Storage and Maintenance
Follow these tips to ensure proper care of your NutropinAq Pen:
- Always keep your NutropinAq Pen and cartridge refrigerated and protected from light when not in use.
- You may remove the pen and cartridge from the refrigerator up to 45 minutes prior to use.
- Do not let your NutropinAq Pen and/or cartridge freeze. Contact your doctor or nurse for a replacement if either the pen or cartridge does not work.
- Avoid excessive temperatures. The solution in the cartridge is stable for up to 28 days after first use when stored at 2-8°C.
- If your pen requires cleaning, do not place under water. Use a damp cloth to wipe away dirt. Do not use alcohol.
- When priming a new cartridge, you may need to repeat Part I, steps 8 and 9 up to a total of 6 times (0.6 mg) to remove air bubbles. Small bubbles may remain and will not affect the dose.
- The pen should contain the NutropinAq that is being used. Do not remove cartridge between injections.
- The NutropinAq cartridge may be used for up to 28 days.
- Do not store the NutropinAq Pen with needle attached.
Published by MIMS March 2023
