Cladribine tablets
Consumer Medicine Information
What is in this leaflet
This leaflet answers some common questions about MOVECTRO.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking MOVECTRO against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may want to read it again.
What MOVECTRO is used for
MOVECTRO is used to treat a type of multiple sclerosis (MS) known as relapsing remitting MS. In this type of MS, MOVECTRO has been shown to result in fewer relapses, less disease activity in the brain and less progression of disability.
The active substance in MOVECTRO is cladribine. Cladribine acts on cells in your immune system, known as lymphocytes, to reduce inflammation in the nervous system caused by MS.
MOVECTRO has been studied for safety and effectiveness when given as 4 treatment courses over 2 years. Until more information is available, you should not receive additional treatment courses during or after the 2 years of treatment.
The long term effects beyond 2 years are still being studied. MOVECTRO may have long term effects that are not yet known, e.g. increased risk of cancer. The effect of MOVECTRO on fertility is unknown. Your doctor is the best person to discuss this with you.
Ask your doctor if you have any questions or concerns about taking MOVECTRO.
This medicine is available only with a doctor’s prescription.
MOVECTRO is not addictive.
Before you take it
When you must not take it
Do not start or continue taking MOVECTRO if:
- You are allergic to cladribine or any of the other ingredients listed at the end of this leaflet
- You have an infection, including any infections which are not active, e.g. chronic hepatitis or tuberculosis
- You have a weakened immune system, e.g. due to a medical condition
- You are taking other medicines that weaken your immune system or affect your bone marrow (e.g. cyclosporin, methotrexate, mitozantrone, azathioprine, natalizumab, or ongoing use of corticosteroids)
- You have moderate or severe kidney or liver disease
If necessary, your doctor can do tests to check your liver or kidney function.
- You have been vaccinated within the last three months with a ‘live’ vaccine, e.g. vaccines for shingles, typhoid, yellow fever, etc
Ask your doctor if you are not sure if you have had a ‘live’ vaccine (see also ‘Using other medicines’ below).
Do not take MOVECTRO if you are pregnant or trying to become pregnant. If you are a man, do not take MOVECTRO if you and your partner are trying to have a baby.
MOVECTRO may harm your baby. You must use reliable methods of contraception to prevent becoming pregnant yourself or making anyone else pregnant.
Your doctor will advise you for how long this is necessary.
Do not take MOVECTRO if you are breastfeeding.
If your doctor believes that MOVECTRO is essential for you, he/she will advise you to stop breastfeeding.
Do not take MOVECTRO after the expiry date printed on the pack.
The expiry date refers to the last day of that month. If you take it after the expiry date has passed, it may not work well.
Do not take MOVECTRO if the packaging shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
Do not give MOVECTRO to a child or adolescent.
There is no experience with its use in children or adolescents under 18 years old.
If you are not sure whether you should start taking MOVECTRO, contact your doctor.
Before you start to take it
Before starting the first course or any subsequent courses of MOVECTRO, tell your doctor about all of your health conditions and have your blood tested as directed by your doctor.
In particular, tell your doctor:
- If you might have an infection
Signs of infection may include fever, chills, sore throat, cough, pain when urinating, or urinating more frequently. If you have any of these or any other signs that make you think you might have an infection or could get an infection, call your doctor as soon as possible. Your doctor will decide whether you can continue treatment or if you should stop for a while.
- If you have or have had cancer
It is not recommended to use MOVECTRO if you currently have cancer. If you had cancer in the past, you should discuss this with your doctor and they can help you decide if MOVECTRO is right for you.
- If you have an intolerance to fructose (a type of sugar)
MOVECTRO contains sorbitol powder. It is not recommended for anyone with fructose intolerance.
If you have not told your doctor about any of the above, tell them before you start or continue taking MOVECTRO.
Also tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Using other medicines
Tell your doctor if you are using any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Do not take MOVECTRO at the same time as any other medicine taken by mouth.
This is because MOVECTRO may interact with other medicines in the stomach. Allow at least 3 hours before and after MOVECTRO when taking other oral medicines.
Tell your doctor if you are or have been treated with:
- Any medicine that weakens your immune system or affects your bone marrow, e.g. cyclosporin, methotrexate, mitozantrone, azathioprine, natalizumab, or ongoing use of corticosteroids
These medicines must not be used together with MOVECTRO (see also ‘When you must not take it’ above).
If considered necessary by your doctor, short-term treatment with corticosteroids is possible with MOVECTRO.
- Any other medicine which may affect the immune system, e.g. interferons, glatiramer acetate
- Any medicine which may affect the blood, e.g. carbamazepine and non-steroid antiinflammatory drugs (NSAIDs)
Your doctor may need to supervise your condition more closely if you are using any of these medicines.
- Certain medicines used to treat the heart, blood, circulation or inflammation, e.g. dipyridamole, nifedipine, nimodipine, cilostazol, dilazep, reserpine or sulindac
These medicines may affect MOVECTRO. Your doctor can advise you.
Tell your doctor if you have been vaccinated within the last 3 months or if you are planning any vaccinations.
MOVECTRO must not be taken within 3 months of vaccination with a ‘live’ vaccine (‘live’ vaccines contain weakened forms of infectious agents). You may need to delay your treatment with MOVECTRO after vaccination and avoid certain vaccinations when taking MOVECTRO. Your doctor can advise you.
Your doctor or pharmacist have more information on medicines to be careful with or avoid while taking MOVECTRO.
Ask your doctor or pharmacist if you have any questions.
How to take it
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
Your doctor will check your blood before you start MOVECTRO and at intervals during and after treatment, to make sure that your treatment can be started or continued.
Make sure that you keep all appointments scheduled for these blood tests.
How much to take
MOVECTRO is administered in treatment courses. A course consists of 1 or 2 tablets taken once a day for 4 or 5 days.
Your doctor will decide the number of tablets per day (1 or 2) and number of treatment days (4 or 5) depending on your body weight.
You will receive a treatment pack that is labelled with the number of tablets you should take each day. You may need to take the same number of tablets each day or some days you might take 2 tablets and then only 1 tablet on the following days. This will be marked on the pack.
Follow the instructions on the pack and ask your doctor or pharmacist if you are unclear about how many tablets to take each day.
How long to take it
Initial Treatment
Initial treatment refers to the first two treatment courses given at the start of a 48-week period.
A treatment course is 1 or 2 tablets each day for 4-5 days. Sometimes the number of tablets will vary from one course to the next.
The second course will usually start 4 weeks after the start date of the first course.
Re-treatment
If your doctor determines it to be appropriate, you will receive a further 2 courses usually 48 weeks and 52 weeks after the first course of the Initial Treatment.
How to take it
Follow closely the ‘Directions for Handling’ included in the pack. Your hands should be dry when handling the tablets.
Swallow the tablet(s) whole with water. Never cut or crush the tablets and do not chew them or allow them to dissolve in your mouth.
Take the tablet(s) immediately after removal from the tablet holder. Do not leave them exposed on surfaces, e.g. on a table, or handle them longer than necessary.
If a tablet is left on a surface or if a broken or fragmented tablet is released from the tablet holder, wash the area thoroughly afterwards.
Wash your hands with soap and water after taking MOVECTRO.
If you lose a tablet, contact your doctor or pharmacist for advice.
When to take it
Take MOVECTRO at about the same time each day. You may take MOVECTRO before or after a meal.
If you forget to take it
If you miss a dose and you remember on the same day you were supposed to take it, take it on that day.
If you miss a dose and do not remember it until the following day, do not take the missed dose along with the scheduled dose.
In this case, take the missed dose on the next day and extend the number of days in that treatment course.
For example: If you forget to take the Day 3 dose and do not remember it until Day 4, take the Day 3 dose on Day 4, and take the Day 4 dose on Day 5. Extend the total number of days in the treatment course by 1 day until the pack is empty.
If you miss 2 consecutive doses (e.g. both Day 3 and Day 4 doses), extend the treatment course by 2 days. In this case, you will take your Day 3 dose on Day 5 and your Day 4 dose on Day 6.
Never take a double dose on the same day to make up for the dose that you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for hints.
If you take too much
Immediately contact your doctor or the Poisons Information Centre (telephone 13 11 26) or go to Accident and Emergency at your nearest hospital if you think you or anyone else may have taken too much MOVECTRO.
Do this even if there are no signs of discomfort or poisoning.
You may need medical attention.
If you take too much MOVECTRO your white blood cells will be affected and you may need additional blood tests. It may also be necessary to stop treatment with MOVECTRO. Your doctor will advise you.
While you are taking MOVECTRO
Things you must do
Keep all your doctor and blood test appointments so that your progress can be checked.
Tell your doctor immediately if you or your partner becomes pregnant during or after your treatment with MOVECTRO.
MOVECTRO may affect the baby if either you or your partner is taking it.
Both men and women must use a proven method of birth control while taking and, for as long as your doctor tells you to, after stopping MOVECTRO.
Usually men should use birth control for at least 3 months, and women for at least 6 months, after their last dose of MOVECTRO.
If you are about to be started on any new medicine or plan to have any vaccinations, tell your doctor or pharmacist that you are taking or have taken MOVECTRO.
‘Live’ vaccines must not be used within 3 months of taking MOVECTRO.
If you are going to have a blood transfusion, tell the medical staff that you are taking this medicine.
Also tell the medical staff if you are undergoing any procedures where a transfusion may be required, e.g. surgery.
Special precautions may be required to prevent an unwanted reaction to the transfusion.
Stay out of the sun as much as possible. If you need to be in the sun, use a sunscreen and wear a hat and shirt to protect your skin from the sun.
Although not known if related to MOVECTRO, skin cancers including melanomas, have been seen in people after treatment. Check your skin regularly and have a doctor check your skin annually for new skin spots or changes to existing spots, moles or freckles.
Things you must not do
Do not stop taking MOVECTRO, or change the dose, without first checking with your doctor.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours or if they have the same condition as you.
Do not use MOVECTRO to treat any other complaints.
Things to be careful of
The effects of your MS or of MOVECTRO may influence your ability to drive a car or operate machinery. For example, MOVECTRO may cause dizziness.
If this happens to you, do not drive or use machines.
Side effects
Tell your doctor as soon as possible if you do not feel well after taking MOVECTRO.
MOVECTRO helps most people with MS, but it may have unwanted side effects in some people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
The most important side effect of MOVECTRO is reduction in number of a type of white blood cell known as lymphocytes. This can occur in more than 30% of patients and may be severe. Older people may be more prone to have this side effect.
Reduced lymphocytes may increase your risk of getting an infection, particularly viral infections.
Contact your doctor as soon as possible if you think you are getting an infection.
Typical infections observed with MOVECTRO include:
- Colds and sore throats
- Vaginal infections
- Shingles
The infection may require treatment, and treatment with MOVECTRO may need to be interrupted until the infection is resolved.
Tell your doctor if you notice any of the following and they worry you:
- Headache
- Dizziness
- Ringing in the ears
- Skin rash or hair loss
- Heavy or irregular menstrual bleeding
These are common side effects of MOVECTRO.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- Signs of allergy such as hives, shortness of breath, wheezing or difficulty breathing, swelling of your face, lips or tongue
- Signs of infection, such as fever, chills, muscle weakness, decreased or difficult urination
- Signs of blood or bone marrow problems, such as extreme fatigue, unusual bruising or bleeding, black or tarry stools
These are serious side effects. If you have them, you may need urgent medical attention or hospitalisation. Serious side effects are rare.
Other side effects not listed above may also occur in some people.
Tell your doctor if you notice anything else that is making you feel unwell.
Do not be alarmed by this list of possible side effects.
You may not experience any of them.
After using it
Storage
MOVECTRO tablets are provided to you in a tablet holder that contains the exact number of tablets you need for a treatment course.
Keep your tablets in the tablet holder until it is time to take them.
This is important for safety reasons, to protect the tablets and because the labelling includes important information.
Keep MOVECTRO in a cool dry place where the temperature stays below 25°C.
Do not store it or any other medicine in the bathroom, near a sink, or on a windowsill. Do not leave it in the car.
Heat and damp can destroy some medicines.
Keep this medicine where young children cannot reach it.
A locked cupboard at least oneandahalf metres above ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking MOVECTRO or if it has passed its expiry date, return it to your pharmacist for disposal.
Medicines should not be disposed of via wastewater or household waste.
Product description
What it looks like
MOVECTRO tablets are white, round, biconvex tablets engraved with ‘C’ on one side and ’10’ on the other side.
Each pack contains 1, 4, 5, 6, 7, 8, 9 or 10 tablets in blister foil within a child-resistant tablet holder.
Ingredients
Each MOVECTRO tablet contains 10 mg of cladribine.
The tablets also contain:
- hydroxypropyl beta cyclodextrin
- sorbitol
- magnesium stearate
MOVECTRO does NOT contain gluten, tartrazine or other azo dyes.
Sponsor
MOVECTRO is made in Italy and supplied in Australia by:
Merck Serono Australia Pty Ltd
3-4/25 Frenchs Forest Road East
Frenchs Forest NSW 2086
For enquiries call 1800 633 463.
The Australian Register Number is AUST R 166483.
This leaflet was updated in November 2010.
DIRECTIONS FOR HANDLING
MOVECTRO® Tablets
It is important to follow these instructions when handling the MOVECTRO tablet holder and taking your tablets. Please also refer to the Consumer Medicine Information before taking MOVECTRO. Consumer Medicine Information is available from your pharmacist.
MOVECTRO is a cytotoxic agent. Do not cut or crush the tablets. Only remove a tablet from the packaging when you are ready to take it and do not leave it sitting on benches or other surfaces. If a tablet is left on a surface or if a broken tablet is released from the blister, the area must be thoroughly washed afterwards.
If your doctor tells you to stop taking MOVECTRO, return any unused product to the pharmacy.
- Have a glass of water ready and make sure your hands are clean and dry before taking the tablet(s).
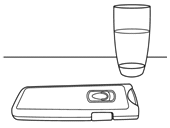
- Turn the tablet holder over to check which day’s tablet(s) you need to take.
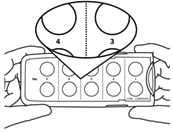
- Pick up the tablet holder in your left hand so that the centre button is facing you and is visible.
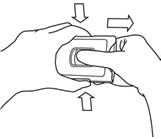
- Pull the centre button back with your right thumb and hold it there.Squeeze the buttons on the sides of the holder in with your left index finger and thumb and keep them squeezed. You can now release the centre button.
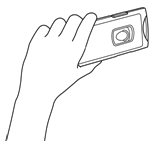
- Pull the tablet tray out with your right hand. You can now stop squeezing the buttons on the sides.
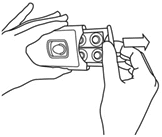
- Push 1 or 2 tablets into your hand with your thumb according to the dose schedule.
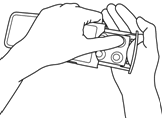
- Put the tablet(s) in your mouth and swallow with water.Tablets must be swallowed whole. Do not chew or allow the tablet to dissolve in your mouth. Contact with skin should be limited to taking the tablet. Avoid touching your nose, eyes, and other parts of the body.
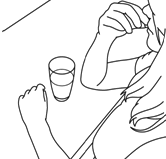
- Push the tablet tray back in until it locks.
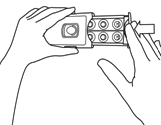
- Wash your hands thoroughly with soap and water.
Keep your tablets in your tablet holder until your next dose. Do not pop the tablets out and store in a different container. Keep the tablet holder in a safe place, out of the reach and sight of children.
Sponsor:
Merck Serono Australia Pty Ltd
Units 3-4, 25 Frenchs Forest Road East
Frenchs Forest NSW 2086
For enquiries call 1800 633 463.
Published by MIMS March 2011
