((frozen) fibrin sealant syringe)
Consumer Medicine Information
What is in this leaflet?
This leaflet answers some common questions about the ARTISS Fibrin Sealant, Two-Component Fibrin Sealant, Deep-Frozen. It does not contain all of the available information. All medicines have risks and benefits. Your doctor has weighed the risks against the benefits for you having ARTISS.
It does not take the place of talking to your doctor or pharmacist. If you have any concerns about having this medicine, ask your doctor or pharmacist.
What ARTISS is used for?
ARTISS is used as a tissue glue to join skin surfaces in burns surgery. ARTISS is also used to join skin surfaces in face-lift surgery.
How does ARTISS work?
The blood coagulation factors in the composition of ARTISS are the same as those found in normal healthy individuals. They are isolated from healthy human plasma, except aprotinin, which is synthetically made. The components are packed in a double-chamber syringe. One of the chambers contains Sealer Protein solution while the other contains Thrombin solution. The double-chamber syringe is stored in a deep frozen state. It will be warmed up to between 33°C -37°C just prior to use.
Before you are given ARTISS
ARTISS should not be given to you if:
- you have a tendency to allergic reactions or hypersensitivity to aprotinin or any of the ingredients in this medicine (listed under What is in ARTISS?). Some of the symptoms of an allergic reaction may include skin rash, swelling of the face, lips or tongue, which may cause difficulty swallowing or shortness of breath.
- the expiry date printed on the pack has passed.
You must tell your doctor if you:
- have any other illness
- are taking any prescription medicine or any other medicines purchased from a pharmacy, health food store or supermarket
- have previously received aprotinin or fibrin sealant, whether you are allergic or not to it
You must tell your doctor if you are pregnant, planning to become pregnant or breast-feeding.
The use of ARTISS during your surgical operation under either of these conditions is not recommended, due to insufficient information supporting such usage. If there is a need to consider the use of this product during pregnancy or breast feeding, your doctor will discuss the risks and benefits with you.
Use in Children
Safety of use in children has been established in procedures to adhere autologous skin grafts in burn patients. Safety of use in children has not been established in patients undergoing skin flaps and grafts during facial plastic and reconstructive surgeries.
How ARTISS is given
How much is given:
Your surgeon will decide how much ARTISS will be given to you, which depends on your need and condition.
How it is given:
ARTISS is for the surgeon’s use during an operation only. Your surgeon will apply it. For health professionals, details are described in the Product Information.
Case of overdose
ARTISS is used only for local application and thus the case of overdose is unlikely to occur.
While you are treated with ARTISS
Discuss with your doctor and surgeon the progress you have experienced after the treatment, especially during the first few days after surgery. As ARTISS is given in a hospital, your healthcare professional will take records of the progress and unexpected reactions.
Side effects
As with any medicine, some side effects may occur. Some patients may have sudden signs of allergy. This is more likely if the product has been used in previous surgery. Your surgeon is aware of this potential adverse effect. If any of the following happen, for example, rash, swelling of the face, lips, mouth or difficulty in breathing, tell your health professional on duty immediately. In exceptional cases symptoms of a serious allergic reaction may occur, known as “anaphylactic shock”. Then, your healthcare professional will take an appropriate action promptly to reverse the symptoms.
You must tell your doctor if you have fever, drowsiness, chills and runny nose followed by rash and joint pain that may develop about two weeks after your surgery. Similarly you must tell your doctor if you have:
- dark urine
- yellowed complexion, feel tired and have low grade fever followed by nausea, vomiting and abdominal pain
- application site irritation
- chest discomfort
- chills
- headache
- lack of energy
- restlessness
- vomiting
Product description
What ARTISS looks like?
It is presented in a preloaded double-chamber syringe package under deep-frozen state.
What is in ARTISS?
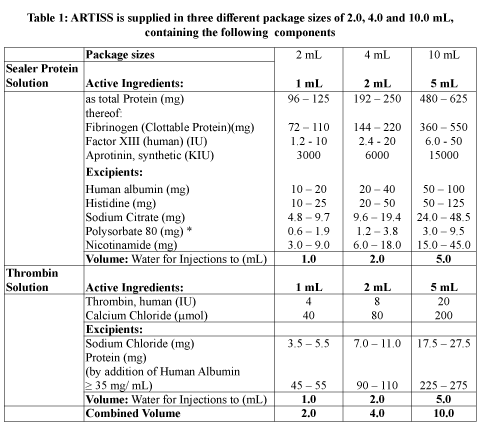
The active components of ARTISS are plasma proteins isolated from pooled human blood of healthy donors according to WHO guidelines. The active components are filled in two separate chambers of a double-chamber syringe, (1) Sealer Protein solution and (2) Thrombin solution, as shown in the following Table 1.
How to store ARTISS
ARTISS is presented in a deep frozen state at –18 °C or colder in a hospital pharmacy. Under these conditions, ARTISS has a shelf life of 2 years. The cold storage chain must not be interrupted until use. Keep container in the outer carton to protect from light.
Unopened pouches, thawed at room temperature, may be stored for up to 14 days at controlled room temperature (not exceeding + 25°C). If not used within 14 days after thawing, ARTISS must be discarded. After thawing, the solution must not be refrigerated or refrozen. ARTISS solutions contain no antimicrobial agent. ARTISS Fibrin Sealant is intended for single use in one patient only and unused solution remaining in the doublechamber syringe should be discarded.
Where can you get more information?
You can get more information from your doctor or pharmacist.
Name and address of the Sponsor
ARTISS, Two component Fibrin Sealant, deep frozen, is manufactured by Baxter AG, Vienna, Austria, and supplied in Australia by:
Baxter Healthcare Pty Limited
1 Baxter Drive,
Old Toongabbie NSW 2146, Sydney
The last revision: 24 June 2013
AUST R 163515
Published by MIMS July 2017
