Contains the active ingredients drospirenone and ethinylestradiol
Consumer Medicine Information
For a copy of a large print leaflet, Ph: 1800 195 055
What is in this leaflet
Read this leaflet carefully before taking your medicine.
This leaflet answers some common questions about drospirenone and ethinylestradiol. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the last page. More recent information on this medicine may be available.
Ask your doctor or pharmacist:
- if there is anything you do not understand in this leaflet,
- if you are worried about taking your medicine, or
- to obtain the most up-to-date information.
You can also download the most up to date leaflet from www.apotex.com.au.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
Pharmaceutical companies cannot give you medical advice or an individual diagnosis.
Keep this leaflet with your medicine. You may want to read it again.
What this medicine is used for
The name of your medicine is YANA®. It contains the active ingredients drospirenone and ethinylestradiol.
This medicine is a combined oral contraceptive, commonly known as a ‘birth control pill’ or ‘the Pill’.
It is used as:
- an oral contraceptive
- treatment of moderate acne vulgaris in women who seek oral contraception
- treatment of symptoms of premenstrual dysphoric disorder (PMDD) in women who have chosen oral contraceptives as their method of birth control.
You may also experience the following benefits:
- improvement in symptoms like bloating, swelling or weight gain related to fluid retention
- more regular and shorter, lighter periods
- a decrease in anaemia (iron deficiency)
- a decrease in period pain.
Some medical conditions such as pelvic inflammatory disease, ovarian cysts, ectopic pregnancy (where the foetus is carried outside of your womb), lumpy breasts and cancer of the uterus (womb) and ovaries may be less common in women using oral contraceptives.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed this medicine for another reason.
This medicine is available only with a doctor’s prescription.
How it works
When taken correctly, it prevents you from becoming pregnant in two ways:
- inhibiting the egg release by stopping it maturing
- changing the cervical mucus consistency, making it more difficult for the sperm to reach the egg.
This medicine has 24 active (hormone) tablets and 4 placebo (inactive) tablets, compared with the traditional 21 active tablets and 7 placebo tablets of other Pills.
This means that with this medicine, you take the active (hormone) tablets for three more days than other oral contraceptive Pills. This helps your hormone levels to stay even.
When the Pill is taken by women under close observation in clinical trials, it is more than 99% effective in preventing pregnancy. However, in real life the Pill is around 92% effective. This is because pills might be missed, or taken with medicines that may interfere with their effectiveness or may not be absorbed due to vomiting and diarrhoea.
Like all oral contraceptives Pills, this medicine is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted infections.
There is no evidence that this medicine is addictive.
Use in children
This medicine should not be used in children.
Before you take this medicine
When you must not take it
Do not take this medicine if:
- You are hypersensitive to, or have had an allergic reaction to, drospirenone and/or ethinylestradiol or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include: shortness of breath, wheezing, difficulty breathing or tightness in chest, swelling of the face, lips, tongue, throat or other parts of the body, rash, itching or hives on the skin.
If you think you are having an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency department at the nearest hospital. - You are taking antiviral medicines which contain glecaprevir, pibrentasvir, sofosbuvir, velpatasvir, voxilaprevir, ombitasvir, paritaprevir or dasabuvir and combinations of these.
These antiviral medicines are used to treat chronic (long-term) hepatitis C (an infectious disease that affects the liver, caused by the hepatitis C virus). - You have or have had a blood clot in:
– the blood vessels of the legs (deep vein thrombosis – DVT)
– the lungs (pulmonary embolism – PE)
– the heart (heart attack)
– the brain (stroke)
– other parts of the body. - You are concerned about an increased risk of blood clots.
Blood clots are rare. Very occasionally blood clots may cause serious permanent disabilities or may even be fatal.
You are more at risk of having a blood clot when you take the Pill. But the risk when taking the Pill is less than the risk during pregnancy. - You are concerned about an increased risk of blood clots because of age or smoking.
The risk of having a heart attack or stroke increases as you get older. It also increases if you smoke. You should stop smoking when taking the Pill, especially if you are older than 35 years of age. - Do not take this medicine if you have, or have had:
– any blood clotting disorders such as Protein C deficiency, Protein S deficiency, Leiden Factor V mutation, Antithrombin III deficiency or other inherited blood clotting conditions
– a confirmed blood test showing:
— increased levels of homocysteine
— antiphospholipid antibodies (APLAs) e.g. anticardiolipin-antibodies and lupus anticoagulant. These may increase your risk for blood clots or pregnancy losses (miscarriage)
– major surgery after which you have not been able to move around for a period of time
– angina pectoris or chest pain
– mini stroke (also known as TIA or transient ischaemic attack)
– severe kidney insufficiency or an acute failure of your kidney
– migraine, accompanied by problems with seeing, speaking or had weakness or numbness in any part of your body
– high risk of blood clots due to conditions such as diabetes mellitus with blood vessel damage, severe high blood pressure or severe high or low level of fats in your blood
– pancreatitis (an inflammation of the pancreas) associated with high levels of fatty substances in your blood
– severe liver disease and your liver function has not returned to normal
– cancer that may grow under the influence of sex hormones (e.g. of the breast or the genital organs)
– benign or malignant liver tumour
– unexplained vaginal bleeding.
If any of these conditions appear for the first time while using the Pill, stop taking it at once and tell your doctor. In the meantime, use non-hormonal (barrier) methods of contraception (such as condoms or a diaphragm). - Do not take this medicine if you are pregnant or think you might be pregnant.
- Do not take this medicine if the expiry date (EXP) printed on the pack has passed.
- Do not take this medicine if the packaging is torn, shows signs of tampering or it does not look quite right.
- Do not give this medicine to a child.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if:
- you smoke
- you or anyone in your immediate family has had blood clots in the legs (DVT) or lungs (PE), a heart attack, a stroke, breast cancer or high cholesterol.
Before you start taking this medicine, tell your doctor if:
- You have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
- You have or have had any medical conditions, especially the following:
- diabetes mellitus
- high blood pressure
- heart valve disorders or certain heart rhythm disorders
- migraine
- an increased potassium blood level (e.g. due to problems with your kidney/s) and also use diuretics or other medicines that may increase the potassium in your blood.
- cancer
- hyperhomocysteinaemia, a condition characterised by high levels of the amino acid homocysteine in the blood.
- intolerance or allergy to lactose.
These tablets contain lactose.
- You are currently breastfeeding or you plan to breastfeed.
Do not take this medicine whilst breastfeeding.
Ask your doctor to check if you have:
- excess weight
- any hereditary or acquired conditions that may make it more likely for you to get blood clots
- high cholesterol or triglycerides
- liver disease
- kidney disease
- high potassium in your blood
- have jaundice (yellowing of the skin) and/or pruritus (itching of the skin) related to cholestasis (condition in which the flow of bile from the liver stops or slows)
- gall bladder disease
- Crohn’s disease or ulcerative colitis (chronic inflammatory bowel disease)
- systemic lupus erythematosus (SLE – a disease affecting the skin all over the body)
- haemolytic uraemic syndrome (HUS – a disorder of blood coagulation causing failure of the kidneys)
- sickle cell disease
- a condition that occurred for the first time, or worsened during pregnancy or previous use of sex hormones (e.g. hearing loss, a metabolic disease called porphyria, a skin disease called herpes gestationis, a neurological disease called Sydenham’s chorea)
- chloasma (yellowish-brown pigmentation patches on the skin, particularly of the face) – if so, avoid exposure to the sun or ultraviolet radiation
- hereditary angioedema – you should see your doctor immediately if you experience symptoms of angioedema, such as swollen face, tongue and/or pharynx and/or difficulty swallowing or hives together with difficulty in breathing.
If any of the above conditions appear for the first time, or recur or worsen while taking this medicine, you should contact your doctor. If you have not told your doctor about any of the above, tell him/her before you start taking this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health foodshop.
Some medicines or foods may interact with drospirenone and/or ethinylestradiol and:
- can have an influence on the blood levels of drospirenone and/or ethinylestradiol
- can make it less effective in preventing pregnancy
- can cause unexpected vaginal bleeding
Such medicines include:
- medicines used to treat tuberculosis such as rifampicin, rifabutin
- medicines used to treat epilepsy such as phenytoin, primidone, barbiturates (e.g. phenobarbitone), carbamazepine, oxcarbazepine, topiramate, felbamate, lamotrigine
- medicines used to treat HIV, such as ritonavir or nevirapine
- some medicines used to treat Hepatitis C Virus (HCV) such as boceprevir, telaprevir, glecaprevir, pibrentasvir, ombitasvir, paritaprevir and dasabuvir
- antibiotics such as penicillin, ampicillin, tetracycline
- macrolide antibiotics (e.g. clarithromycin, erythromycin)
- medicines used to treat fungal infections, such as ketoconazole and griseofulvin
- ciclosporin, a medicine used for suppressing the immune system
- medicines used to treat high blood pressure, chest pain and/or irregular heartbeats such as diltiazem, verapamil
- etoricoxib, an anti-inflammatory medicine used to treat pain
- tizanidine, melatonin or midazolam which are medicines that relax the body
- theophylline, a medicine that helps with breathing
- herbal medicines containing St John’s wort
- grapefruit juice.
These medicines may be affected by YANA® or may affect how well it works. If you are taking any of these your doctor may need to alter the dose of these medicines or prescribe a different medicine.
You might have an increase in potassium in the blood if you are taking this medicine with medicines that may increase potassium levels in the blood. These include:
- medicines used to treat high blood pressure, such as ACE inhibitors, angiotensin-II-receptor antagonists and diuretics
- certain anti-inflammatory medicines, such as indomethacin
- aldosterone antagonists, such as spironolactone and eplerenone.
In a study of women taking drospirenone together with an ACE inhibitor, no significant differences were observed in the potassium levels when compared to the placebo.
You may need to use additional barrier methods of contraception (such as condoms or a diaphragm) while you are taking any of these medicines and for some time after stopping them.
Your doctor will be able to advise you about how long you will need to use additional contraceptive methods.
Your doctor and pharmacist have more information on medicines that you need to be careful with or avoid while taking this medicine.
Other medicines not listed above may also interact with drospirenone and ethinylestradiol.
How to take this medicine
Follow carefully all directions given to you by your doctor or pharmacist. Their instructions may be different to the information in this leaflet.
How to take it
Take one tablet daily at about the same time every day. You must take this medicine every day even if you do not have sex very often. It will also help you remember when to take it.
Swallow the tablets whole with water.
It does not matter if you take this medicine before or after food.
Take your first pink (active) tablet from the green area on the blister pack corresponding to the day of the week. Follow the direction of the arrows on the blister pack until all the tablets have been taken. Each blister pack is marked with the day of the week.
If you do not understand the instructions on the blister pack, ask your doctor or pharmacist for help.
A period should begin 2-3 days after starting to take the white inactive tablets and may not have finished before the next pack is started.
Always start a new blister pack on the same day of the week as your previous pack.
Taking this medicine for the first time
If you are starting this medicine after a natural cycle, and you have not used a hormonal contraceptive in the past month, start on the first day of your period, i.e. on the first day of your menstrual bleeding.
You may also start on days 2-5 of your period, but in that case make sure you also use additional barrier contraceptive precautions (e.g. condom) for the first 7 days of tablet-taking.
Your doctor will advise you when to start if you
- are taking this medicine after having a baby
- have had a miscarriage or an abortion.
This medicine is not recommended if you are breastfeeding.
Changing from another contraceptive
Changing from a combined oral contraceptive:
Start taking this medicine on the day after taking the last active tablet in your previous Pill pack.
A withdrawal bleed may not occur until the end of the first pack of this medicine.
You can also switch to this medicine after taking one or more inactive tablets in your previous Pill pack, but no later than the day after taking the last inactive tablet.
Ask your doctor or pharmacist if you are not sure which were the active/placebo tablets in your previous Pill pack. Your previous Pill pack may have different colour tablets to those of this medicine.
Changing from a progestogen-only pill (‘minipill’):
Stop taking the minipill on any day and start taking this medicine at the same time the next day after you took your last minipill.
You must also use additional barrier contraceptive precautions when having intercourse (e.g. condoms or a diaphragm) for the first 7 days of tablet-taking.
Changing from a progesterone-only injectable, implant or intrauterine system (IUS):
Start taking this medicine when your next injection is due, or on the day that your implant or IUS is removed.
You must also use additional barrier contraceptive precautions when having intercourse (e.g. condoms or a diaphragm) for the first 7 days of tablet-taking.
Changing from a vaginal ring:
Start on the day of removal of the ring but at the latest when the next application would have been due.
How long to take it for
You can stop taking this medicine at any time. If you are considering becoming pregnant, it is recommended that you begin taking a vitamin supplement containing folic acid. It is best that you start taking folic acid tablets before you stop taking this medicine and not stop until your doctor advises this. Seek advice from your doctor or pharmacist about suitable supplements. It is both safe and recommended that you take folic acid during pregnancy.
If you want to delay a period
To delay your period,
- continue taking the pink active tablets in the current blister,
- skip the white placebo tablets in the last row of the same blister, and
- start a new blister by taking the pink active tablet from the green area corresponding to the day of the week (to ensure that you take your tablets on the corresponding day of the week as marked on the pack, you may have some extra tablets left over in your current blister which you can discard).
You can continue to delay your period by skipping the white placebo tablet in the second blister. The delay can be extended until the last pink active tablet in the third blister is taken.
If you wish for your period to begin at any time during the extension, stop taking the pink active tablets and start taking the white placebo tablets instead.
You should get your period approximately 2 – 3 days after you start taking the white placebo tablet. After taking the last white placebo tablet, start a new blister by taking the pink active tablet.
During the extension, you may have some breakthrough bleeding or spotting on active tablet-taking days.
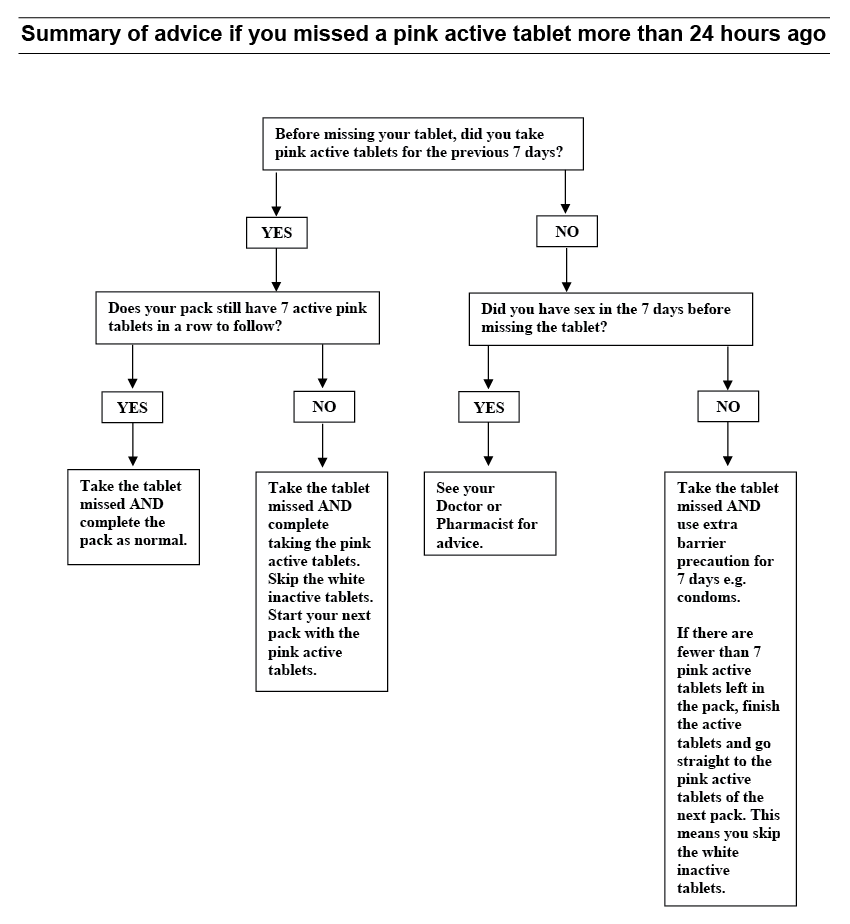
If you forget to take it
If you miss a tablet and take the missing tablet within 24 hours of missing it, you will be protected against pregnancy. If you are more than 24 hours late, follow these detailed instructions:
For this medicine to be most effective, pink active tablets need to be taken uninterrupted for 7 days.
If you have been taking the pink active tablets for 7 uninterrupted days and miss a pink active tablet, take the missed tablet as soon as you remember, then go back to taking your medicine as you would normally, even if this means taking two tablets in one day. You will be protected against pregnancy.
The chance of pregnancy after missing a pink active tablet depends on when you missed the tablet. There is a higher risk of becoming pregnant if you miss a tablet at the beginning or end of a pack.
If after taking your missed tablet you have less than 7 days of pink active tablets left in a row, you should finish the active tablets in your pack, but skip the white placebo tablets and start a new pack.
This is the best way to maintain contraceptive protection. However, you may not have a period until the end of the pink active tablets of the second pack. You may have spotting or breakthrough bleeding on tablet-taking days.
If you have been taking the pink active tablets for less than 7 days and miss a pink active tablet, take the missed tablet as soon as you remember, then go back to taking your medicine as you would normally, even if this means taking two tablets in one day. In addition, you should also use additional barrier contraceptive precautions (e.g. condoms or a diaphragm) for the next 7 days.
If you have had sexual intercourse during that time, there is a possibility of pregnancy and you may need emergency contraception.
If you forget to take more than one pink active tablet, seek advice from your doctor or pharmacist about what to do.
If you have had sexual intercourse in the week before missing your tablets, there is a possibility of becoming pregnant.
If you forget to take a white placebo tablet, you do not need to take it later because they do not contain any active ingredients. However, it is important that you discard the missed white tablet(s) to make sure that the number of days between taking active tablets is not increased as this would increase the risk of pregnancy. Continue with the next tablet at the usual time. Ask your doctor or pharmacist to answer any questions you may have.
Please see the diagram at the end of this leaflet for ‘Summary of advice if you missed a pink active tablet more than 24 hours ago’.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively, go to the Accident and Emergency department at your nearest hospital. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take several pink active tablets at once, you may feel sick or vomit or may bleedfrom the vagina. Even girls who have not yet started to menstruate but have accidentally taken this medicine may experience such bleeding.
While you are taking this medicine
Things you must do
Tell any doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Have regular check-ups with your doctor.
When you are taking the Pill, your doctor will tell you to return for regular check-ups including getting a Cervical Screening Test. Your doctor will advise how often you need a Cervical Screening Test. A Cervical Screening Test can detect abnormal cells lining the cervix. Sometimes abnormal cells can progress to cancer.
If you are about to start on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Stop taking this medicine and see your doctor immediately if you notice any of the following signs:
- one-sided swelling of the leg and/or foot or along a vein in the leg
- pain or tenderness in the leg which may be felt only when standing or walking
- increased warmth in the affected leg; red or discoloured skin on the leg
- sudden onset of unexplained shortness of breath or rapid breathing
- sudden coughing or coughing up of blood
- sharp chest pain or sudden severe pain in the chest which may increase with deep breathing
- severe light headedness or dizziness
- rapid or irregular heartbeat
- sudden pain, swelling and slight blue discoloration of an extremity
- sudden numbness or weakness of the face, arm or leg, especially on one side of the body
- sudden trouble walking, dizziness, loss of balance or coordination
- sudden confusion, slurred speech or aphasia
- sudden partial or complete loss of vision, double vision, painless blurring of vision which can progress to loss of vision
- sudden, severe or prolonged headache with no known cause
- loss of consciousness or fainting with or without seizure
- pain, discomfort, pressure, heaviness, sensation of squeezing or fullness in the chest, arm, or below the breastbone
- discomfort radiating to the back, jaw, throat, arm, stomach
- feeling of being full, having indigestion or choking
- sweating, nausea, vomiting
- extreme weakness and anxiety.
If you are going to have surgery, tell the surgeon or anaesthetist beforehand that you are taking this medicine.
The risk of having blood clots is temporarily increased as a result of major surgery, any surgery to the legs or pelvis, neurosurgery or major trauma. In women who take the Pill, the risk may be higher.
In women at risk of prolonged immobilisation (including major surgery, any surgery to the legs or pelvis, neurosurgery, or major trauma), your doctor may tell you to stop taking (in the case of elective surgery at least four weeks in advance) and not resume until two weeks after complete remobilisation. Another method of contraception should be used to avoid unintentional pregnancy. Your doctor may prescribe other treatment (e.g. treatment for blood clots) if your medicine has not been discontinued in advance.
If you notice possible signs of a blood clot, stop taking the Pill and consult your doctor immediately.
Other risk factors for blood clotting include temporary immobilisation including air travel of greater than 4 hours, particularly in women with other risk factors. Consult your doctor if you plan to air travel for greater than 4 hours.
Consult your doctor if you develop high blood pressure while taking this medicine – you may need to stop taking it.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you vomit within 3-4 hours or have severe diarrhoea after taking a pink active tablet, the active ingredients may not have been completely absorbed. This is like missing a tablet. Follow the advice for missed tablets.
If you have unexpected bleeding and it continues, becomes heavy, or occurs again, tell your doctor. When taking these tablets for the first few months, you can have irregular vaginal bleeding (spotting or breakthrough bleeding) between your periods. You may need to use sanitary protection but continue to take your tablets as normal. Irregular vaginal bleeding usually stops once your body has adjusted to the Pill, usually after about 3 months.
If you have missed a period, but you have taken all your tablets, it is very unlikely that you are pregnant, as long as:
- you have taken the pink active tablets at the right time
- you have not been taking medicine(s) that may interfere with this medicine
- you have not vomited or had severe diarrhoea during this cycle.
If this is so, continue to take this medicine as usual. If you have any concerns consult your doctor or pharmacist.
If you miss your period twice in a row, you may be pregnant even if you have taken the Pill correctly. Stop taking this medicine and seek advice from your doctor. You must use a non-hormonal method of contraception, (such as condoms or a diaphragm) until your doctor rules out pregnancy. Do not start the next pack of this medicine until your doctor has checked that you are not pregnant.
If you choose to delay your period while taking this medicine, your regular bleeding is not expected to occur during the extension period when the intake of the pink active tablet is uninterrupted. Therefore, the absence of regular bleeding cannot be used as a sign of an unexpected pregnancy and as such, unexpected pregnancy may be difficult to recognise. Although pregnancy is unlikely if this medicine is taken as directed, if for any reason you think you might be pregnant, contact your doctor and do a pregnancy test. This may be of particular importance if you are also using other medications since some medications are known to be harmful to the foetus.
This medicine will not protect you from HIV-AIDS or any other Sexually Transmitted Infections (STIs), such as chlamydia, genital herpes, genital warts, gonorrhoea, hepatitis B, human papilloma virus and syphilis.
To protect yourself from STIs, you will need to use additional barrier contraceptives (e.g. condoms).
Things you must not do
Do not:
- Give this medicine to anyone else.
- Take this medicine to treat any other condition unless your doctor tells you to.
- Stop taking your medicine, or change the dosage, without first checking with your doctor. You may become pregnant if you are not using any other contraceptive and you stop taking this medicine, or do not take a tablet every day.
Things to be careful of
Be careful when driving or operating machinery until you know how this medicine affects you.
Side effects
Tell your doctor as soon as possible if you do not feel well while you are taking this medicine or if you have any questions or concerns.
This medicine helps most people, but it may have unwanted side effects in a few people.
Do not be alarmed by the following lists of side effects. You may not experience any of them. All medicines can have side effects. Sometimes they are serious but most of the time they are not. You may need medical attention if you get some of the side effects.
Tell your doctor if you notice any of the following.
This list includes the more common side effects. Mostly, these are mild and lessen with time:
- nausea
- headache, including migraines
- mood changes, including depression
- unscheduled vaginal bleeding
- abnormal periods
- irregular bleeding between periods
- breast tenderness or pain
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
These are very serious side effects and you may need urgent medical attention or hospitalisation. These side effects are usually rare:
- pain in the chest, arm or below the breastbone
- pain or discomfort radiating to the back
- breathlessness and/or difficulty breathing
- swelling, pain or tenderness of one leg
- sudden weakness, numbness or bad ‘pins and needles’ of the face, arm or leg, especially on one side of the body
- severe, sudden stomach pains
- sudden trouble walking, dizziness, loss of balance or coordination
- a fainting attack, or you collapse
- unusual headaches or migraines that are worse than usual
- sudden problems with your speech, understanding or eyesight.
The side effects listed above are possible signs of a blood clot (thrombosis).
- jaundice (yellowing skin or yellowing eyes)
- you cough up blood
- breast lumps
- unexplained vaginal bleeding.
Tell your doctor or pharmacist if you feel unwell. Other side effects not listed above may occur in some patients.
Blood clots and the Pill
Blood clots may block blood vessels in your body. This type of blood clot is also called thrombosis. Blood clots sometimes occur in the deep veins of the legs (deep venous thrombosis (DVT)). If a blood clot breaks away from the veins where it has formed, it may reach and block the blood vessels of the lungs, causing pulmonary embolism (PE).
Blood clots can also occur in the blood vessels of the heart (causing a heart attack) or the brain (causing a stroke).
Blood clots are a rare occurrence and can develop whether or not you are taking the Pill. They can also happen during pregnancy. The risk of having blood clots is higher in Pill users than in non-users, but not as high as during pregnancy.
The excess risk of a blood clot is highest during the first year after a woman takes the Pill for the first time or after having a break from the Pill for 4 weeks or more.
If you notice possible signs of a blood clot, stop taking this medicine and consult your doctor immediately.
To prevent pregnancy, you must also use additional barrier contraceptive precautions (e.g. condoms or a diaphragm).
If you are concerned about an increased risk of blood clots while on this medicine, speak to your doctor.
Cancer and the Pill
Breast cancer has been diagnosed slightly more often in women who take the Pill than in women of the same age who do not take the Pill.
This slight increase in the numbers of breast cancer diagnoses gradually disappears during the course of the 10 years after women stop taking the Pill.
It is not known whether the difference is caused by the Pill. It may be that these women were examined more often, so that the breast cancer was noticed earlier.
It is important that you check your breasts regularly and contact your doctor if you feel any lumps.
In rare cases benign liver tumours and, even more rarely, malignant liver tumours have been reported in users of the Pill. These tumours may lead to internal bleeding.
Contact your doctor immediately if you have severe pain in your abdomen.
Cervical cancer has been reported to occur more often in women who have been using the Pill for a long time. This finding may not be caused by the Pill but may be related to sexual behaviour and other factors.
Allergic reactions
If you think you are having an allergic reaction to drospirenone and ethinylestradiol, do not take any more of this medicine and tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- cough, shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
- fainting
- hay fever-like symptoms.
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it.
If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or it has passed its expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
What YANA® looks like
Active tablet: Plain, round, pink, film coated tablet.
Placebo tablet: Plain, round, white, film coated tablet.
This medicine comes in a box containing either 1, 3 or 6* blister packs. Each blister pack contains 24 pink active tablets and 4 white placebo tablets.
* Not all pack sizes may be available.
Ingredients
Each pink active tablet contains 3 mg of drospirenone and 20 µg of ethinylestradiol as the active ingredients.
It also contains the following inactive ingredients:
- lactose monohydrate
- pregelatinised maize starch
- povidone
- croscarmellose sodium
- polysorbate 80
- magnesium stearate
- OPADRY II complete film coating system 85F34610 Pink.
Each white placebo tablet contains the following inactive ingredients:
- lactose
- povidone
- magnesium stearate
- OPADRY II complete film coating system 85F18422 White.
This medicine contains lactose.
This medicine is gluten-free, sucrose-free, tartrazine-free and free of other azo dyes.
Australian Registration Numbers
YANA® drospirenone 3 mg and ethinylestradiol 20 microgram film-coated tablet (blister pack):
AUST R 200633.
Distributor
Arrotex Pharmaceuticals Pty Ltd
15 – 17 Chapel St,
Cremorne VIC 3121
YANA® is a registered trade mark of Apotex Pty Ltd.
This leaflet was last updated in:
July 2023
Published by MIMS November 2023




